本批舒肺方NAC為2022年製造,包裝工廠誤植效期兩年為07/2024,實際原料效期經檢驗為三年,應為07/2025,以上請知悉。
現貨有限,售完為止。
Pure NAC 成份是N乙烯半胱氨酸,是氨基酸(蛋白質的最小單位),除了明顯的酸味,也會有蛋白質特殊的味道!
舒肺方 (Pure NAC) 的主成份是N-乙醯半胱氨酸,這是半胱氨酸的乙醯化衍生物,它一
在2021年6月一份研究指出,
原文資料摘自: https://pubmed.ncbi.nlm.nih.gov/34182881/
在2021年12月一份臨床報告指出,
因此,我們建議使用口服NAC來預防和改善新冠COVID-
舒肺方的保健目的:
● 增強天然免疫機能
● 幫助肝臟和腎臟解毒功能
● 保護肝臟和腎臟避免受到病毒或毒素破壞
● 超級抗氧化劑,可抗發炎,抗癌,抗過敏
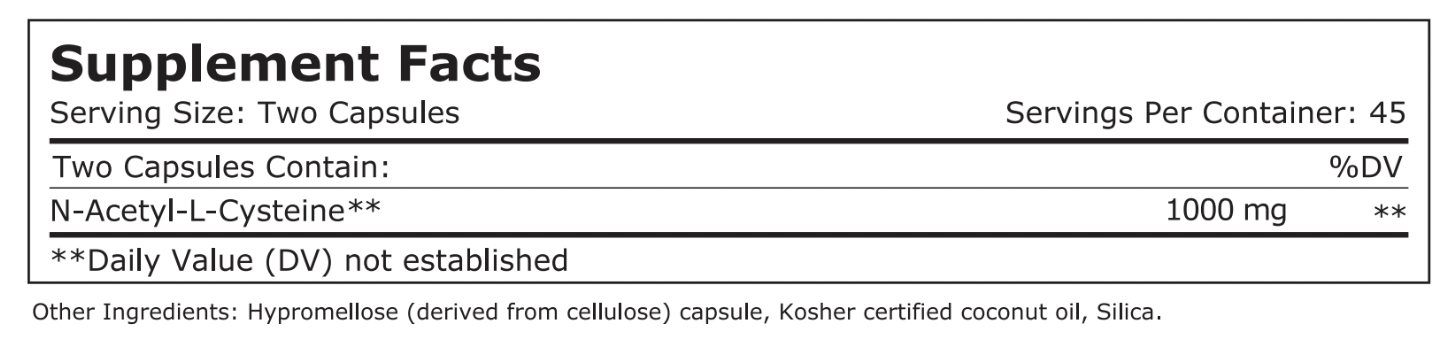
建議使用劑量:
● 預防保養 每日1 顆
● 新冠肺炎或呼吸道疾病患者 每日2-3顆
N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study
Abstract
Background: N-acetyl-cysteine (NAC) has been previously shown to exert beneficial effects in diverse respiratory diseases, through antioxidant and anti-inflammatory actions. Our aim was to evaluate NAC potential impact in hospitalised patients with COVID-19 pneumonia, in terms of progression to severe respiratory failure (SRF) and mortality.
Patients and methods: This retrospective, two-centre cohort study included consecutive patients hospitalised with moderate or severe COVID-19 pneumonia. Patients who received standard of care were compared with patients who additionally received NAC 600 mg bid orally for 14 days. Patients' clinical course was recorded regarding (i) the development of SRF (PO2/FiO2 <150) requiring mechanical ventilation support and (ii) mortality at 14 and 28 days.
Results: A total of 82 patients were included, 42 in the NAC group and 40 in the control group. Treatment with oral NAC led to significantly lower rates of progression to SRF as compared to the control group (p < .01). Patients in the NAC group presented significantly lower 14- and 28-day mortality as compared to controls (p < .001 and p < .01 respectively). NAC treatment significantly reduced 14- and 28-day mortality in patients with severe disease (p < .001, respectively). NAC improved over time the PO2/FiO2 ratio and decreased the white blood cell, CRP, D-dimers and LDH levels. In the multivariable logistic regression analysis, non-severe illness and NAC administration were independent predictors of 28-days survival.
Conclusion: Oral NAC administration (1200 mg/d) in patients with COVID-19 pneumonia reduces the risk for mechanical ventilation and mortality. Our findings need to be confirmed by properly designed prospective clinical trials.
Keywords: ARDS; COVID-19; N-acetyl-cysteine; antioxidant; mortality; pneumonia.

